Innovative
Solutions,
Analytical
Precision.
Solutions,
Analytical
Precision.
Our commitment to advancing pharmaceutical quality and safety through cutting-edge analytical methods.

Empowering
Innovations,
Ensuring
Quality.
Innovations,
Ensuring
Quality.
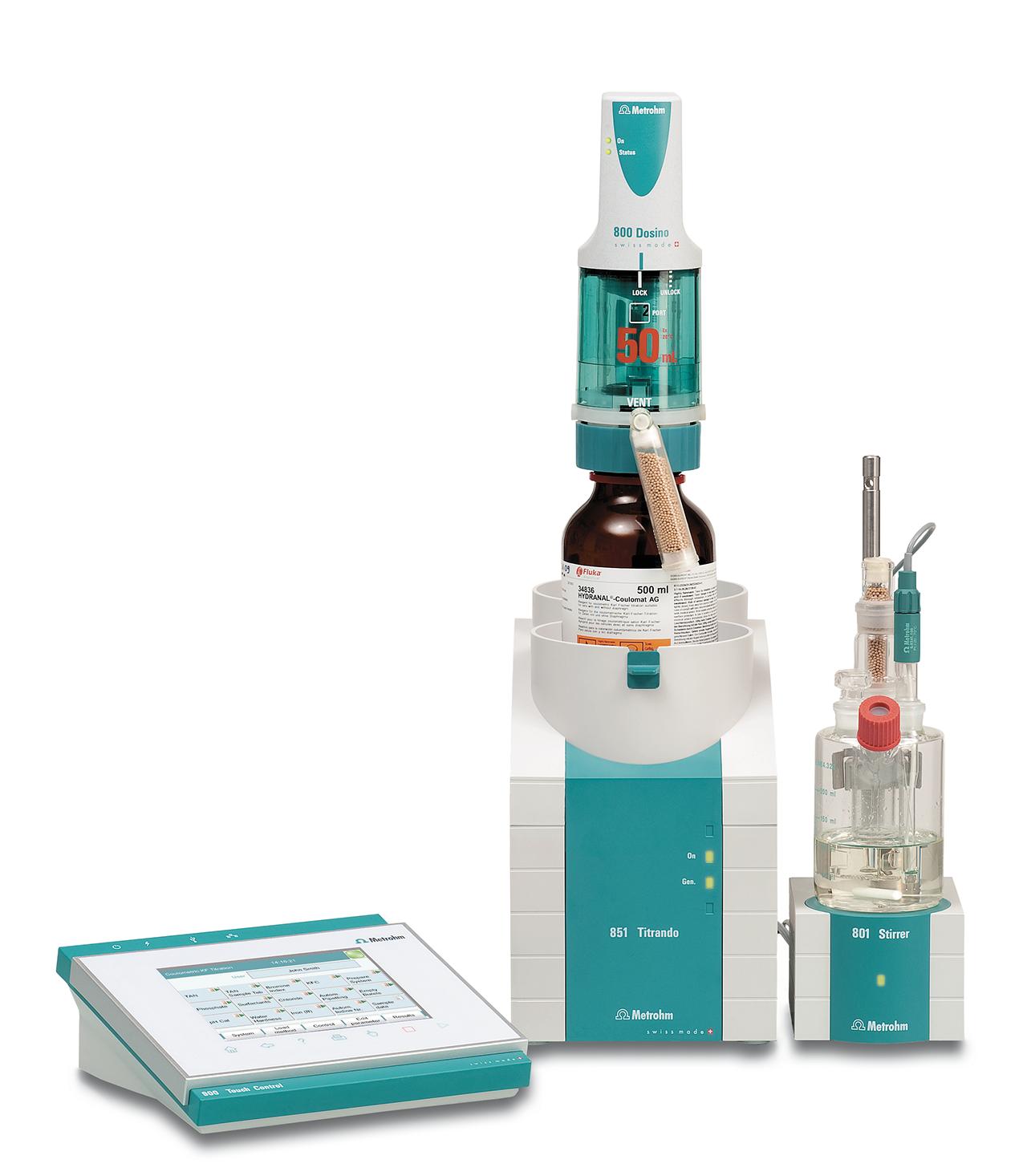
Comprehensive Solutions Catering to the Pharmaceutical Sector